Abstract

INTRODUCTION: High dose cytarabine (HiDAC) for acute myeloid leukemia (AML) consolidation has historically been given as an inpatient (IP) regimen. Reasons for IP administration may include regimen scheduling/logistics, hydration requirements, patient assessments, and adverse effect management. However, the landscape of oncology treatment is moving towards chemotherapy administered in an outpatient (OP) setting. This may be directed by hospital census, reimbursement challenges, or patient satisfaction. At The Ohio State University, IP-HiDAC was identified as one regimen to transition to the OP setting. We present a detailed description of the process and our institutional experience by which patients receive OP-HiDAC through clinic and ambulatory home infusion.
METHODS: To develop a safe process for patients to receive OP-HiDAC, we created a guideline authored by pharmacists, physicians, and a clinical nurse specialist. Exclusion criteria for OP-HiDAC includes serum creatinine >1.5 mg/dL, enrollment on a clinical trial requiring IP administration, and lack of social support. To facilitate standardization, an order-set was developed for all required supportive care prescriptions. Providers are required to have a visit with OP-HiDAC candidates the week prior to chemotherapy initiation to educate and organize the logistics of OP treatment.
For OP-HiDAC, cytarabine is given over 2 hours every 10 hours on days (D) 1, 3, and 5 every 28-day cycle. The morning dose is given in the OP clinic and the evening dose is administered at home through an ambulatory infusion pump. Pharmacists and nurses are required to provide education prior to or during the first infusion appointment, including reviews of the home pump disconnect process and medication calendars. Caregivers can choose to disconnect the chemotherapy at home after the evening infusion, or patients can return to the clinic on D2, 4, and 6 for pump disconnect. Caregivers are required to complete a signature, speech, and gait log 1 hour prior to each evening dose. Patients are able to choose whether to self-inject growth factor on D7 (filgrastim, peg-filgrastim, or biosimilars) or return for clinic administration, with insurance approval.
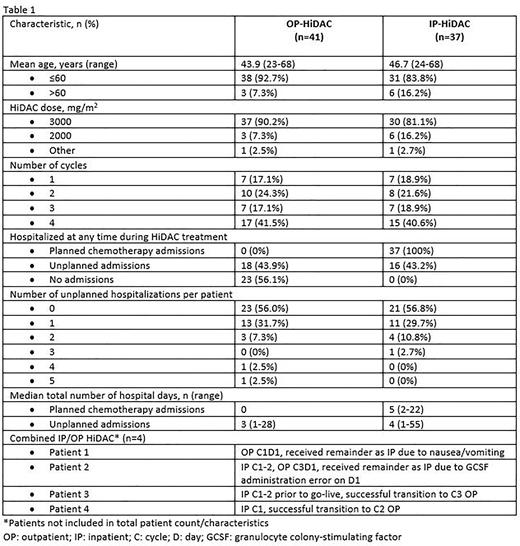
RESULTS: A retrospective chart review characterized our experience and healthcare resource utilization (HRU) of HiDAC from June 1, 2020 (OP-HiDAC go-live) through July 2022, see Table 1. During the first month, only 1 patient was treated outpatient each week, with the remainder of patients receiving IP-HiDAC. This process allowed for workflow evaluation from staff, patients, and caregivers. Since making OP-HiDAC the preferred regimen, 41 patients received OP-HIDAC and 37 received IP-HiDAC. Common reasons for IP-HIDAC included lack of caregiver, infection/bleeding concern, and lack of transportation. In the OP-HiDAC and IP-HiDAC groups, the majority of patients were ≤60 years old, 93% and 84%, respectively, and received full dose 3000 mg/m2/dose, 90% and 81%, respectively. In the IP-HiDAC group, 16 patients (43%) had an unplanned hospital admission(s) during treatment, compared to 18 patients (44%) in the OP-HiDAC group. Common reasons for unplanned admissions in both groups were comparable and included febrile neutropenia, sepsis, thrombocytopenia with bleeding (including hematuria and epistaxis), pain, syncope, and dyspnea. One OP-HIDAC patient had suspected neurotoxicity and after a brief admission for monitoring, continued therapy OP without recurrence of symptoms. Another OP-HiDAC patient had a pump malfunction on C2D1 in which the evening dose did not infuse. The caregiver correctly notified triage and was given further instructions, preventing a hospital admission. The median total number of hospital days for unplanned admissions with OP-HiDAC and IP-HiDAC were 3 and 4, respectively, suggesting that OP-HiDAC patients did not require longer hospitalizations for toxicity or complications.
CONCLUSIONS: OP-HiDAC through clinic and ambulatory pump home infusion is an exciting alternative to IP-HiDAC, saving inpatient resource utilization by avoiding planned chemotherapy admissions. OP-HiDAC requires significant education for both healthcare teams and patients/caregivers to be successful and ultimately can provide opportunity for increased patient satisfaction with less time spent in a hospital or clinic setting.
Disclosures
Mims:Daiichi Sankyo: Other: Data Safety and Monitoring Board; Jazz Pharmaceuticals: Other: Data Safety and Monitoring Board; Zentalis: Membership on an entity's Board of Directors or advisory committees; Ryvu: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Syndax: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal